Requirements under the Cannabis Act and the Cannabis Regulations
Health Canada is the federal department responsible for helping the people of Canada maintain and improve their health. Health Canada is committed to improving the lives of all of Canada's people and to making this country's population among the healthiest in the world as measured by longevity, lifestyle and effective use of the public health care system.
Disclaimer: This document does not constitute part of the Cannabis Act or its regulations. It should be read in conjunction with the relevant sections of the Act and its regulations. The information in this document is not intended to substitute for, supersede or limit the requirements under the legislation. In the event of discrepancy between the legislation and this document, the legislation shall prevail.
The reader is advised to consult other legislation that may apply to them or their activities, such as applicable provincial or territorial legislation.
This document may be updated from time to time so the reader is encouraged to check back periodically.
The reader is advised to consult other legislation that may apply to them or their activities, such as application provincial or territorial legislation.
This document may be updated from time to time so the reader is encouraged to check back periodically.
1.0 Purpose
This guide provides guidance and information about the packaging and labelling requirements for cannabis products under the Cannabis Act (also referred to in this guide as "the Act" and abbreviated to CA for references) and the Cannabis Regulations (also referred to in this guide as "the Regulations", and abbreviated to CR for references).
2.0 Background
The Cannabis Regulations sets out requirements pertaining to how cannabis and cannabis products must be packaged and labelled prior to sale, distribution or export. Specifically, the Regulations require plain packaging and labelling for all cannabis products with restrictions on logos, colours, and branding. Cannabis products must be in packaged in a child-resistant container and be labelled with the standardized cannabis symbol, the mandatory health warning message, and include specific product information (e.g., brand name of the cannabis product, class of cannabis, delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) information, licence holder information). These measures aim to reduce the risks of accidental consumption and overconsumption as well as reduce the appeal of cannabis products to young persons while providing consumers with the information they need to make informed decisions before using cannabis.
Licence holders are responsible for complying with the Act and Regulations, and other legislation that may apply to them or their activities. Health Canada does not review or pre-approve packages and labels of cannabis products.
Health Canada applies a risk-based approach to compliance and enforcement whereby risk pertains to health, safety and the credibility of the regulatory system, among other factors. The Compliance and enforcement policy for the Cannabis Act can be found on Health Canada's website.
3.0 Scope
This guide applies to cannabis products that are sold or distributed in Canada.
Cannabis products do not include cannabis or a cannabis accessory that contains cannabis that is intended for an animal, or a drug containing cannabis.
This guide does not apply to industrial hemp or hemp seed derivatives.
This guide does not apply to shipping containers such as outer wrappings or boxes used to transport cannabis products, or a crate in a transport truck.
The information in this guide is based on the Cannabis Regulations, as amended bythe Regulations Amending the Cannabis Regulations (New Classes of Cannabis) which were published in the Canada Gazette, Part II, on June 26, 2019 and will come into force on October 17, 2019. The amended Regulations include transitional provisions related to packaging and labelling. More information is outlined in section 9.0 of this guide.
On its website, Health Canada publishes other guidance documents and information that may be used in conjunction with this guide to support compliance with the Act and its regulations. For consistency and transparency, this guide and other guidance documents and information are updated as required to reflect changes to policies and/or operations.
The examples and figures in this guide are for illustrative purposes only and do not represent actual cannabis products.
4.0 Definitions and abbreviations
4.1 Definitions
The Cannabis Act and the Cannabis Regulations should be referred to for definitions. The definitions in this section are provided for ease of reference.
Brand element: As defined in the Cannabis Act, includes a brand name, trademark, tradename, distinguishing guise, logo, graphic arrangement, design or slogan that is reasonably associated with, or that evokes,
- cannabis, a cannabis accessory or a service related to cannabis; or
- a brand of any cannabis, cannabis accessory or service related to cannabis.
Cannabis accessory: As defined in the Cannabis Act, means
- a thing, including rolling papers or wraps, holders, pipes, water pipes, bongs and vaporizers, that is represented to be used in the consumption of cannabis; or
- a thing that is deemed under subsection 2(3) of the Act to be represented to be used in the consumption of cannabis.
Cannabis product: As defined in the Cannabis Regulations, means cannabis of only one of the classes that are set out in Schedule 4 to the Cannabis Act-or a cannabis accessory that contains such cannabis-after it has been packaged and labelled for sale to a consumer at the retail level. It does not include cannabis that is intended for an animal or a cannabis accessory that contains cannabis that is intended for an animal, or a drug containing cannabis.
Common name: As defined in the Cannabis Regulations for the purposes of its Part 7, in respect to edible cannabis, has the same meaning as in subsection B.01.001(1) of the Food and Drug Regulations.
Common Names for Ingredients and Components Document: The document entitled Common Names for Ingredients and Components, prepared by the Canadian Food Inspection Agency and published on its website, as amended from time to time.
Exterior display surface: As defined in the Cannabis Regulations for the purposes of its Part 7, means the area on the exterior surface of an immediate container to which a label is applied and that is visible under customary conditions of purchase or use.
Immediate container: As defined in the Cannabis Regulations, means a container that is in direct contact with cannabis or a cannabis accessory that is a cannabis product or, if a wrapper is in direct contact with the cannabis or the cannabis accessory, with the wrapper.
Label: As defined in the Cannabis Act, includes a legend, word or mark that is, or is to be applied or attached to or included in, or that accompanies or is to accompany, cannabis or a cannabis accessory or a package.
Package: As defined in the Cannabis Act, means any inner or outer container or covering.
Point: As defined in the Cannabis Regulations, means the unit of measurement for type size that is known as a PostScript point and is equal to 0.3527777778 millimetres.
Principal display panel: As defined in the Cannabis Regulations for the purposes of its Part 7, has the same meaning as in subsection 2(2) of the Consumer Packaging and Labelling Regulations. Specifically it means:
- in the case of a container that is mounted on a display card, that part of the label applied to all or part of the principal display surface of the container or to all or part of the side of the display card that is displayed or visible under normal or customary conditions of sale or use or to both such parts of the container and the display card,
- in the case of an ornamental container, that part of the label applied to all or part of the bottom of the container or to all or part of the principal display surface or to all or part of a tag that is attached to the container, and
- in the case of all other containers, that part of the label applied to all or part of the principal display surface
4.2 Abbreviations
- CA
- Cannabis Act
- CBD
- cannabidiol
- CFIA
- Canadian Food Inspection Agency
- CR
- Cannabis Regulations
- EU
- European Union
- FDA
- Food and Drug Act
- FDR
- Food and Drugs Regulations
- g
- gram
- INCI
- International Nomenclature of Cosmetic Ingredients
- mg
- milligram
- ml
- millilitre
- PDP
- principal display panel
- ppm
- parts per million
- SFCR
- Safe Food for Canadians Regulations
- THC
- delta-9-tetrahydrocannabinol
- THCA
- delta-9-tetrahydrocannabinolic acid
- TVPA
- Tobacco Vaping Products Act
- μg/g
- microgram per gram
5.0 Prohibitions on packaging and labelling of cannabis
The Cannabis Act and Cannabis Regulations outline a number of prohibitions related to the packaging and labelling of cannabis and cannabis products. Unless authorized under the Act, the prohibitions listed in this subsection apply to all cannabis and cannabis products.
5.1 General prohibitions
Under the Cannabis Act, it is prohibited for a person that is authorized to sell cannabis (or for a person that sells a cannabis accessory) to sell it in a package or with a label:
- That does not meet the requirements of the Regulations [25, CA]
- If there are reasonable grounds to believe that a package or label could be appealing to young persons [26(a), 27(a), CA]
- That sets out a testimonial or endorsement, however displayed or communicated [26(b), 27(b), CA]
- That sets out a depiction of a person, character or animal, whether real or fictional [26(c), 27(c), CA]
- That associates it or any one of its brand elements with, or evokes a positive or negative emotion about or image of, a way of life such as one that includes glamour, recreation, excitement, vitality, risk or daring [26(d), 27(d), CA]
5.2 Other prohibited representations
The prohibitions listed in this subsection apply to all cannabis products including the package, label or panel of a container.
Under the Regulations, it is prohibited to make an express or implied representation, including by way of a brand element, if there are reasonable grounds to believe that the representation could:
- Associate the cannabis product with a tobacco product as defined in section 2 of the Tobacco Vaping Products Act (TVPA), or a vaping product to which that Act applies [132.32, CR].
- Associate the cannabis product with an alcoholic beverage [132.31, CR].
- Create the impression that health or cosmetic benefits may be derived from the use of the cannabis product This prohibition does not apply to drugs containing cannabis that are health products (i.e. prescription drugs containing cannabis or medical devices which are approved under the Food and Drug Act (FDA) [104.12(2), 132.28, CR]). For more information, refer to the FDA and its regulations as well as the Guidance on health products containing cannabis or for use with cannabis.
Additionally, for edible cannabis, it is prohibited to make an express or implied representation, including by way of a brand element that would:
- Concern the energy value referred to in item 2 of the table to 132.22 of the Regulations or the amount of nutrient referred to in items 3 to 15 of that table or in items 5 to 37 of the table to section B.01.402 of the Food and Drugs Regulations (FDR) [132.29(1), CR].
- Cause reasonable grounds to believe that the representation could create the impression that the cannabis product is intended to meet the particular dietary requirements of an individual who has a physical or psychological condition as a result of a disease, disorder or injury, or for whom a particular effect, including weight loss, is to be obtained by a controlled intake of food, or of individuals who are under 18 years of age [132.3, CR].
Additionally, for cannabis extracts, it is prohibited to display an indication or illustration, including a brand element that could:
- Cause a person to believe that the cannabis product has a flavour set out in column 1 of the Schedule 3 to the TVPA, other than the flavour of cannabis, such as confectionary, dessert, soft drink and energy drink flavours [132.13, CR].
5.3 False, misleading or deceptive information
It is prohibited for a person that is authorized to sell cannabis (or for a person who sells a cannabis accessory) to sell it in a package or with a label that contains any information that is false, misleading or deceptive or that is likely to create an erroneous impression about the:
- Characteristics, value, quantity, composition, strength, concentration, potency, purity, quality, merit, safety, health effects or health risks of the cannabis [26(e), CA].
- Design, construction, performance, intended use, characteristics, value, composition, merit, safety, health effects or health risks of a cannabis accessory [27(e), CA].
6.0 General packaging and labelling requirements
The requirements of the Regulations result in plain packaging and labelling for all cannabis products. This includes restrictions on logos, colours, and branding as well as specific display formats about how product information must appear on the label (e.g., type style, size, and spacing). These measures are designed to:
- Reduce the attractiveness and appeal of cannabis products, particularly to young persons
- Make the standardized cannabis symbol and health warning messages more prominent and noticeable
- Provide consumers with accurate information about the content and use of the cannabis product
Plain packaging and labelling applies to all surfaces, including labels and the various types and parts of the package such as containers, wrappers, and coverings (e.g., shrink wrap).
| Cannabis Regulations reference | Requirement | Notes |
|---|---|---|
|
113 |
Single uniform colourFootnote1
|
Colour may be different for each surface (e.g., exterior, interior) and panel. The exterior of any container and the interior surface of immediate containers may be a metallic colour if that surface is made of metal, excluding the label or image. |
|
115 |
Smooth textureFootnote1
|
Does not apply to features necessary to facilitate opening and closing of the container and those to assist persons who are visually impaired. |
|
116(1) |
No hidden features including
|
|
|
116(2) |
No features that can change surface area including
|
Does not apply to wrappers. |
|
117 |
Not emit scent or sound |
|
|
121 |
No cut-out windows |
Does not apply to coverings or wrappers. |
|
111 |
No brand element |
Subject to specific provisions of the Regulations. See sections 8.1.4 and 8.1.5 of this guide. |
|
112 |
No image and/or informationFootnote1
|
A container may display a bar code but it must only appear once. It must be in a rectangle that is black and white without any other images or designs. A wrapper must display the standardized cannabis symbol. See section 8.1.1 of this guide for more information about the symbol. |
|
18 |
No inserts or leafletsFootnote1 |
Cannabis products may be accompanied by the document entitled Consumer Information - Cannabis. There is an exception that allows additional information to be included with any shipment, including shipments sent to registered patients. |
Tip: Colours that have the lustre of metal or metallic properties include Pantone Metallics or Pantone Premium Metallics. Fluorescent properties include pigments that absorb ultraviolet energy and transmit it as a longer wavelength, such as the Pantone 800 series.
7.0 Packaging requirements
All cannabis products must be packaged in an immediate container. Some products may also have an outer container in addition to the immediate container, while other products may have shrink wraps or wrappers (e.g., foil wrappers around chocolate).
Where a cannabis product has multilayered packaging, all elements must be packaged and labelled according to the regulatory requirements.
In addition to the general packaging and labelling requirements outlined in section 6.0 of this guide, the Regulations also outline specific requirements related to the different parts of a package.
7.1 Immediate container requirements
As mentioned, all cannabis products must be packaged in an immediate container. This container is the part of the package that is in direct contact with the cannabis or the cannabis accessory. The immediate container may also be in direct contact with the wrapper. Table 2 summarizes the immediate container requirements.
| Cannabis Regulations reference | Requirement | Notes |
|---|---|---|
|
1(2) |
Cannot contain more than one class of cannabis
|
Applies to the outermost container as well. |
|
108(b),(c) |
Prevent contamination of the cannabis
|
Does not apply to cannabis plants. |
|
108(a) |
Be opaque or translucent |
Does not apply to cannabis plants and seeds. |
|
108(e) |
Be child resistant
|
Does not apply to cannabis plants and seeds. |
|
108(d) |
Have a security feature
|
Does not apply to cannabis plants and seeds. |
|
108(f) |
Not exceed the maximum amount of cannabis
|
With the exception of cannabis plants, refer to Schedule 3 to the Act for equivalency values for different cannabis classes. |
|
96(1) |
Not exceed the maximum THC quantity
|
The quantity of THC mentioned here takes into account the potential to convert THCA into THC. |
|
122.3 |
Control measures for cannabis extracts not in discrete units
|
The quantity of THC mentioned here takes into account the potential to convert THCA into THC. Cannabis extract in liquid form mentioned here must be at a temperature of 22 ± 2°C. |
7.2 Outermost container requirements
The outermost container in which a cannabis product is packaged must not contain:
- Food;
- More than one class of cannabis set out in Schedule 4 to the Act; or
- More than one immediate container [122.4(1), CR].
There are some exceptions to multiple immediate containers for edible cannabis that are further detailed in section 7.4 of this guide.
7.3 Food grade packaging requirements
There are food grade packaging requirements for:
- The immediate container in which the edible cannabis, or a cannabis accessory that contains edible cannabis is packaged;
- Any wrapper that is direct contact with the edible cannabis, or a cannabis accessory that contains edible cannabis; and
- Any wrapper that is in direct contact with a cannabis extract intended for ingestion, or a cannabis accessory that contains cannabis extract intended for ingestion.
Division 23 of Part B of the FDR, among other things, prohibits the sale of foods in packages that may impart any substance to the contents which may be harmful to the consumer of the food.
Section 186(a)(i),(ii), and (v) to (vii) of the Safe Food for Canadians Regulations (SFCR) outline requirements for a package including to be clean, suitable for its intended use, and free from odours that might affect the food [122.2, CR].
Important: Wrappers may only be used if they are required to maintain the quality or stability of the cannabis product.
In these cases, the wrapper must be in direct contact with the cannabis (or the cannabis accessory) and with one or both of the following:
- The immediate container of the cannabis product
- A wrapper that in direct contact with the cannabis (or the cannabis accessory) [122.1, CR]
7.4 Co-packaging edible cannabis
The use of multiple immediate containers in an outermost container for edible cannabis is allowed provided that all requirements related to how these containers are packaged and labelled are met [122.4, CR].
Each immediate container must:
- Meet the immediate container requirements outlined in section 7.1 this guide (e.g., single uniform colour, child resistant, security feature) [122.4(2)(a), CR]
- Contain edible cannabis with consistent properties including size [122.4(2)(f), CR]
- Be labelled in accordance with the requirements for discrete units or non-discrete units as applicable [122.4(2)(b), CR]
The outermost container must:
- Meet the requirements for discrete units and the word "unit" must be interpreted as the immediate container. As such, for instances, the outermost container must be labelled with the quantity of THC and CBD in each immediate container, rather than the quantity for eachdiscrete unit in each container [132.18, 122.4(3), CR]
- Not contain, in total, a quantity of THC that exceeds 10 mg in the immediate containers, taking into account the potential to convert THCA into THC [122.4(2)(c), CR]
- Not contain, in total, a quantity of cannabis in the immediate containers that exceeds the equivalent of 30 g of dried cannabis as determined in accordance with Schedule 3 to the Act [122.4(2)(d), CR]
- Be labelled with the statement "Contains the equivalent of ## g of dried cannabis" where ## is the equivalent quantity of dried cannabis, in grams, as determined in accordance with Schedule 3 to the Act [122.4(2)(e), CR]
Tip: There may be requirements pertaining to the use of certain containers (e.g., hazard symbol on pressurized containers). The Consumer Chemicals and Containers Regulations, for instances, should be referred to for more information.
Important: The Government of Canada recognizes that plastic pollution is a growing problem in Canada and around the world.
To help reduce the amount of waste created by cannabis product packaging, the Regulations permit wrappers and peel back-type labels as well as flexibility for packaging materials other than plastics (e.g., cardboard).
Health Canada encourages the use of innovative and environmentally sound packaging approaches, provided the requirements in the Regulations are satisfied.
8.0 Labelling requirements
The Regulations prescribe what, where and how information must appear on the label of all cannabis products. This includes strict requirements on the presentation of information such as type style and size, colours, and spacing.
All information on the label must be:
- In English and French, except for International Nomenclature of Cosmetic Ingredients (INCI) names and European Union (EU) trivial names [130(1), CR]
- Clear, prominently displayed and legible under normal conditions of purchase and use [130(2), CR]
A label should be either printed on, or securely applied to a package (e.g., as a sticker). It should not fall off or be easily removed during transportation or normal use of the product.
8.1 Required information on the principal display panel of the label
Every label must have a principal display panel (PDP) that must appear on the principal display surface of the container. This is the surface that is displayed or is visible under normal or customary conditions of purchase or use. As defined in section 2 of the Consumer Packaging and Labelling Regulations, the PDP of the label may be all or part of the principal display surface.
| Part of container that is visible under normal conditions of purchase or use | Area of principal display surface |
|---|---|
|
Side or surface |
Total area of such side or surface (excluding the top) |
|
No side or surface |
Any 40% of the total surface area that can be displayed (excluding the top and bottom) |
|
Lid |
Total area of the top surface of the lid |
|
Bag with sides of equal dimensions |
Total area of one of the sides |
|
Bag with sides of more than one size |
Total area of one of the largest sides |
Important: If there are separate PDPs to display English and French information, each PDP of the label must be properly labelled.
The subsections below outline the requirements of the PDP of a label:
8.1.1 Standardized cannabis symbol
The standardized cannabis symbol must be displayed on the PDP of the label and is used to inform and warn consumers that the cannabis product contains THC. It is incorporated by reference as part of the Regulations and must appear on the label of all cannabis products containing THC in a concentration greater than 10 μg/g, taking into account the potential to convert THCA into THC [123(1)(f), 130(5), CR]. A high-resolution format of the standardized cannabis symbol must be downloaded from Health Canada's website: Standardized cannabis symbol
Tip: Documents "Incorporated by reference" are documents not in the text of the regulations that are made part of the regulations. Documents incorporated by reference have the force of law and can be updated from time to time.
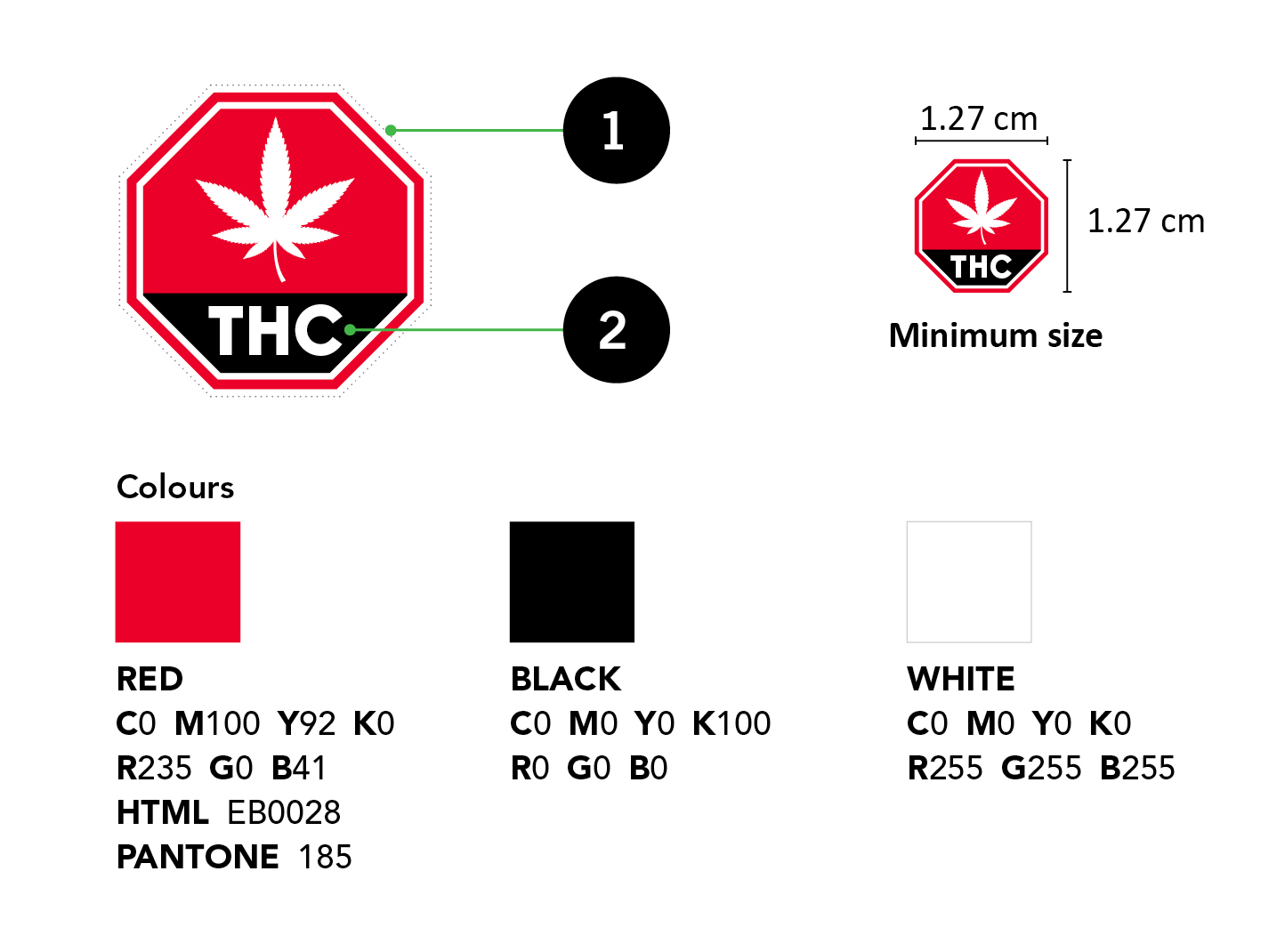
he standardized cannabis symbol is a red octagon. There is a white border on the octagon. Inside the octagon, there is a white cannabis leaf which covers two thirds of the inside of the shape. Underneath the cannabis leaf, one third of the octagon is filled with black and contains the letters THC in a white, bold, uppercase font. There is a dotted line around the octagon to show the outset that is required.
The standardized cannabis symbol is shown in a large size and a smaller size. The text underneath the smaller size says "Minimum size". The minimum dimensions of the symbol shows that it must be at least 1.27 centimetres in width by 1.27 centimetres in height.
The Colours
There is a red square that illustrates the red colour to be used for the standardized cannabis symbol. The description to the right is as follows:
RED
C0 M100 Y92 K0
R235 G0 B41
HTML EB0028
PANTONE 185
There is a black square that illustrates the black colour to be used for the standardized cannabis symbol. The description underneath is as follows:
BLACK
C0 M0 Y0 K100
R0 G0 B0
HTML 000000
There is a white square that illustrates the black colour to be used for the standardized cannabis symbol. The description underneath is as follows:
WHITE
C0 M0 Y0 K0
R255 G255 B255
HTML FFFFFF
- White border of at least 2 points on all sides (dotted border shown not required, used to illustrate outset).
- Oriented in such a manner that its text is readable from left to right when the container is displayed or visible under the customary conditions of purchase and use.
Note: The standardized cannabis symbol must appear in the upper left 25% of the principal display panel. If the size of the symbol is changed, its dimension must remain proportional vertically and horizontally.
Important: If the size of the symbol is changed, its dimensions must remain proportional vertically and horizontally [130(5)(e), CR].
It is prohibited to display on the label a representation such as an image, sign, mark or symbol that resembles or can be mistaken for the standardized cannabis symbol [131, CR].
8.1.2 Health warning messages
The health warning message must be displayed on the PDP of the label and is comprised of a primary and secondary message in a yellow box to inform and warn consumers of the potential health risks and effects of using cannabis [123(1)(e), 130(6)-(7), CR]. These messages are incorporated by reference as part of the Regulations and can be obtained from Health Canada's website: Cannabis health warning messages
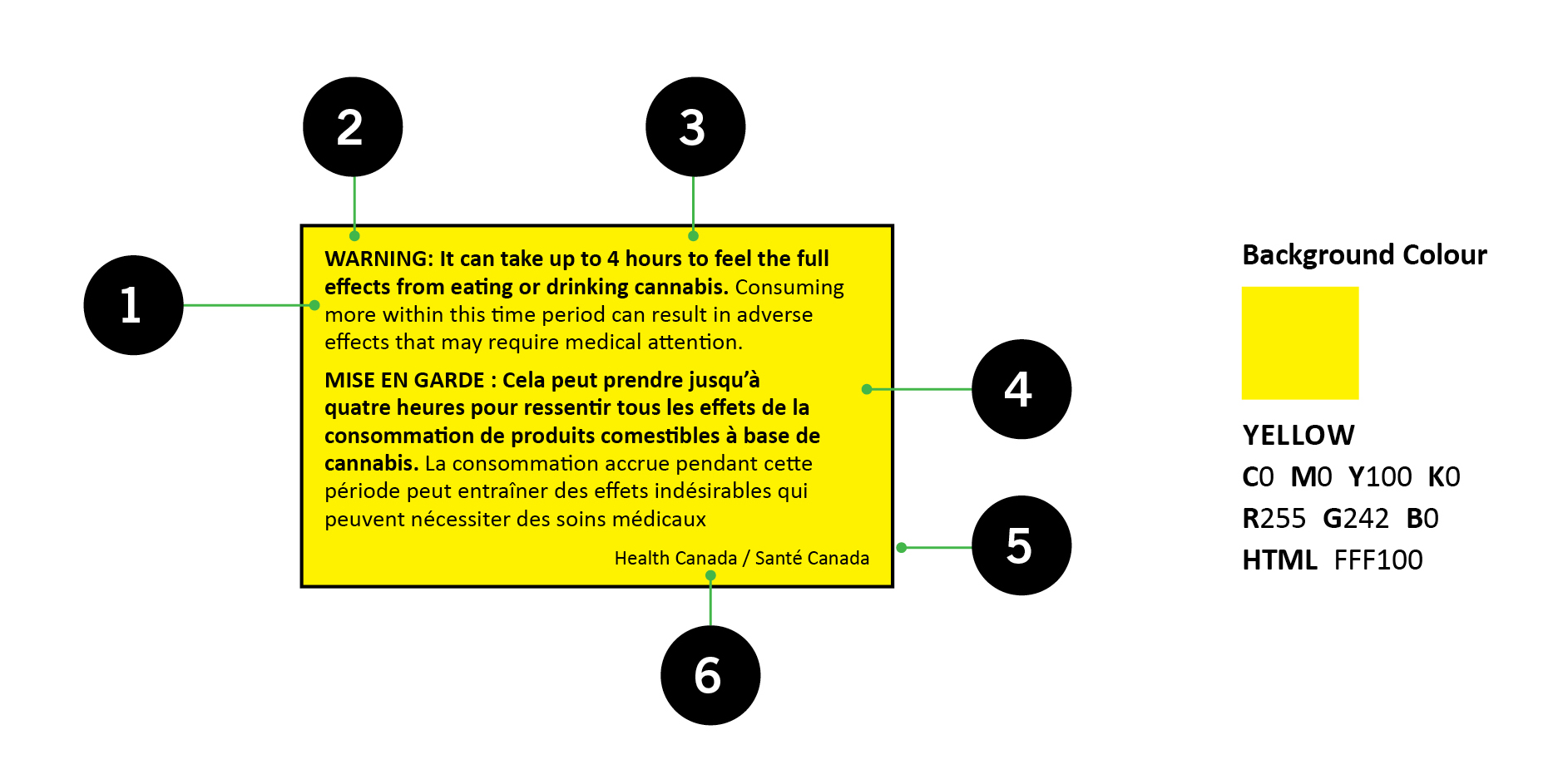
Figure 2 - Text Equivalent
Important: An updated list of health warning messages will take effect when the amended Regulations come into force on October 17, 2019.
The health warning messages must be displayed in rotation on each type of container of each brand name of the cannabis product that is packaged in a year, so that each message is displayed, to the extent possible, on equal numbers of containers of that product [123(4), CR]. Cannabis topicals are exempted from this requirement as they have a single health warning message.
8.1.3 THC and CBD content
The unit of measure and whether the THC and CBD content is represented by quantity (mg) or concentration (mg/g) can vary depending on the class of cannabis in the immediate container, whether the cannabis product is in discrete units or not and the intended use of the cannabis product [130(3)(e), CR]. Table 3 summarizes how this information must be displayed on the PDP of the label:
| Cannabis Regulations reference | Class of cannabis | In discrete units | Not in discrete units | |
|---|---|---|---|---|
|
124 |
Dried or |
Not intended for inhalation |
Intended for inhalation |
THC ## mg/g |
|
132.1 |
Cannabis extract |
Not intended for inhalation |
Intended for inhalation |
THC ## mg/g |
|
132.15 |
Cannabis topical |
THC per unit ## mg or mg/g |
THC ## mg or mg/g |
|
|
132.18 |
Edible cannabis |
THC per unit ## mg |
THC ## mg |
|
|
Note: ## used as a placeholder for numerical values |
||||
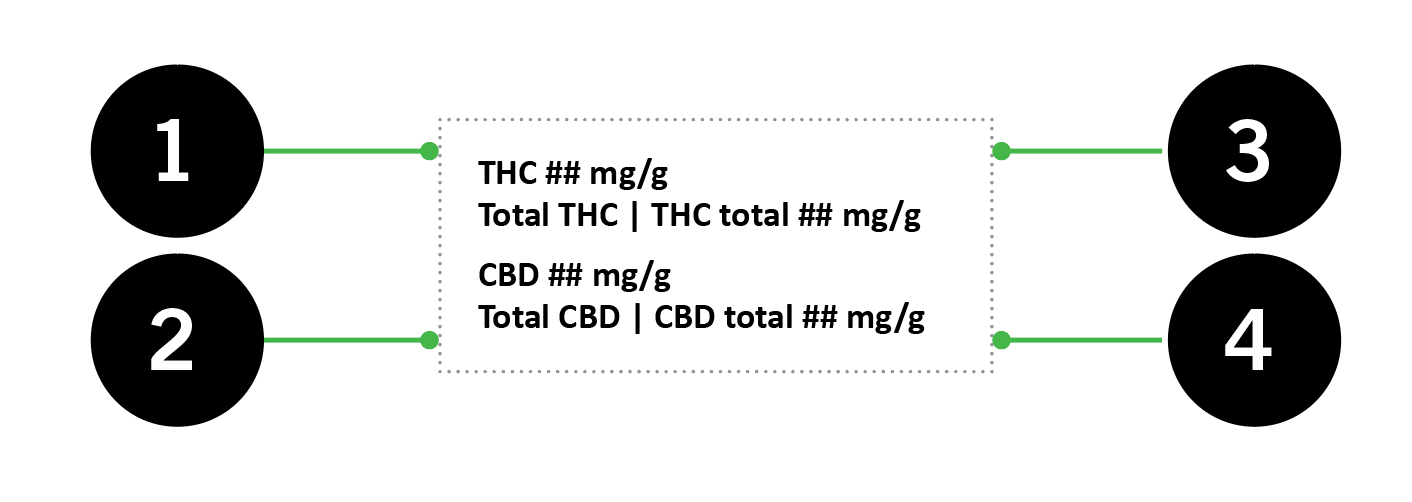
Figure 3 - Text Equivalent
Important: Table 2 in section 7.1 of this guide lists the maximum THC quantities allowed in an immediate container by class of cannabis. Additionally, the label of a cannabis product cannot include a total quantity of THC taking into account the potential to convert THCA into THC that exceeds:
- 10 mg per unit for dried or fresh cannabis or cannabis extract (in discrete units and not intended for inhalation) [124(2), 132.1(2), CR]
- 10 mg per activation for cannabis extracts in non-discrete units with an integrated dispensing device [132.12(2), CR]
- 10 mg for edible cannabis [132.18(2), 132.19(2), CR]
The THC and CBD content values displayed on the label must be the values calculated when testing for the levels of cannabinoids. Further requirements related to composition testing are found in sections 90 and 92 of the Regulations as well as the Good production practices guide for cannabis.
8.1.4 Brand name
The brand name of the cannabis product must be displayed on the PDP of the label; however it must not be in metallic or fluorescent colours. It can be in any type style but the type size must be smaller than or equal to the type size used for the health warning message that appears on the label [130(4), CR].
8.1.5 An additional brand element (optional)
Only one other brand element (in addition to the brand name) may be displayed on the PDP of the label. This element could include, for example, a slogan or logo. It must not be in metallic or fluorescent colours.
If the brand element is an image or graphic, the surface area must be smaller than or equal to the surface area of the standardized cannabis symbol that appears on the label.
If the brand element is text only, the type size must be smaller than or equal to the type size used for the health warning message that appears on the label.
For cannabis products containing less than 10 μg/g THC which are not required to display the standardized cannabis symbol, the brand element, if an image, must be smaller than or equal to 25% of the PDP of the label, and smaller than or equal to the surface area of the health warning message that appears on the label [130(9), CR].
8.1.6 International radiation symbol (if applicable)
If edible cannabis is irradiated, the international radiation symbol set out in subsection B.01.035(5) of the FDR with a statement such as "irradiated", "treated with radiation" or "treated by irradiation" must be displayed on the PDP of the label [102.6, 130(11), CR]. The symbol can be found on the Canadian Food Inspection Agency's (CFIA) website: Food Irradiation
8.2 Required information about the cannabis product
The Regulations require that all cannabis products be labelled with specific product information, and this information must be displayed on either the PDP or the area of the label that is visible under customary conditions of purchase and use (i.e., the exterior display surface). Table 4 summarizes the required information by class of cannabis.
The information must be presented in both English and French in the following format [130(3)(a)-(d), CR]:
- Background: white
- Type colour: black
- Type style: regular weight and width standard sans serif without italics
- Type size: at least 6 points and must be smaller than the type size used for the health warning message that appears on the label
- Outset: at least 6 points from the white background on all sides
- Leading: at least 7 points
| Cannabis Regulations Reference | Requirement | Class of cannabis | ||||
|---|---|---|---|---|---|---|
| Cannabis plants or plant seeds | Dried or fresh cannabis | Cannabis extract | Cannabis topical | Edible cannabis |
||
|
123(1)(a) |
Contact information of the licence holder: name, telephone number, email address |
Required |
Required |
Required |
Required |
Required |
|
123(1)(b) |
Class of cannabis: one of the classes that are set out in Schedule 4 to the Cannabis Act |
Required |
Required |
Required |
Required |
Required |
|
123(1)(c)(ii) |
Lot number: information related to the manufacturing stage of the product; must be preceded by Lot number, Lot no., Lot, or (L) (e.g., Lot 12345) |
Required |
Required |
Required |
Required |
Required |
|
123(1)(c)(iii) |
Recommended storage conditions: may refer to elements of temperature, light conditions or humidity (e.g., store in a dry place, avoid direct light) |
Required |
Required |
Required |
Required |
Required |
|
123(1)(c)(iv) |
Packaging date: the date the cannabis product was packaged on. It should be the same date as required in the record document [224(2)(b), 225(2)(b), CR]. (e.g., Packaged on 2019 AL 21) |
Required |
Required |
Required |
Required |
Required |
|
105(1) |
Expiry date: the end date of the stability period of the cannabis product, if any, must include at least a month and year (e.g., Expiry date 2019 AL) or a statement that no expiry date has been determined. |
Not required |
Required |
Required |
Required |
Not required |
|
124(1)(a) |
Net weight of the cannabis: the net weight of cannabis expressed in numerical values followed by grams. (e.g., Net weight 100 g). The net weight must be in the tolerance limits set out in the document entitled Tolerance Limits for the Net Weight and Volume Declared on Cannabis Product Labelling. |
Required |
Required |
Required |
Required |
Required |
|
132.18(1)(a) |
Net volume of the edible cannabis: the net volume of the edible cannabis not in solid form expressed in numerical values followed by millilitres. May be expressed in ml, mL or mℓ. (e.g., Net volume 100 ml). The net volume must be in the tolerance limits set out in the document entitled Tolerance Limits for the Net Weight and Volume Declared on Cannabis Product Labelling. |
Not required |
Not required |
Not required |
Not required |
Required |
|
124(1)(b) |
Number of discrete units, if applicable: (e.g., 10 capsules, 10 chocolates) |
Required |
Required |
Required |
Required |
Required |
|
100 |
Net weight per discrete unit, if applicable: the net weight of cannabis (in grams) per discrete unit (e.g., 10 g per capsule) |
Required |
Required |
Required |
Required |
Not required |
|
123(1)(g) |
Cannabis possession statement: "Contains the equivalent of ## g of dried cannabis" where ## is the equivalent quantity of dried cannabis, in grams, as determined in accordance with Schedule 3 to the Act. |
Required for cannabis plant seeds but not required for cannabis plants |
Required for fresh cannabis but not required for dried cannabis |
Required |
Required |
Required |
|
123(d) |
The warning statement: "KEEP OUT OF REACH OF CHILDREN / TENIR HORS DE LA PORTÉE DES ENFANTS" in upper case font |
Required |
Required |
Required |
Required |
Required |
|
132.1(1)(j) |
Identity by common name of product or its function: generic name of product; may be the name of the cannabis product form (e.g., cream, drops, capsule, chocolate) |
Not required |
Not required |
Required |
Required |
Required |
|
124(1)(h) |
Intended use of cannabis product: intended uses such as for inhalation, ingestion, for use on skin |
Not required |
Required |
Required |
Required |
Not required |
|
132.2 |
Durable life date: required for edible cannabis that has a durable life date of 90 days or less, or may be on the label if date is more than 90 days; must include the words "Best before" in the format year-month-day where the year is optional (e.g., Best before 19 JN 28) |
Not required |
Not required |
Not required |
Not required |
Required |
|
132.1(1)(h) |
List of ingredients (and constituents): must show, fully and accurately all of the ingredients in the cannabis product. See sections 8.2.1 to 8.2.5 of this guide. |
Not required |
Not required |
Required |
Required |
Required |
|
132.1(1)(i) |
Sources of food allergen or gluten, and added sulphites: must be identified in the list of ingredients or in a statement. This includes any cross-contamination statements, if applicable. See section 8.2.6 and 8.2.7 of this guide. |
Not required |
Not required |
Required for allergens only |
Not required |
Required |
|
132.18(1)(n) |
Nutrition facts table: must be displayed in the format set out in the document entitled Directory of Nutrition Facts Table Formats for Edible Cannabis. See section 8.2.2 of this guide. |
Not required |
Not required |
Not required |
Not required |
Required |
Tip: The durable life date must use the following bilingual symbols to display months [132.2 (2), CR]. These symbols may be used to display the month in the expiry date and packaging date.
JA : January
FE : February
MR : March
AL : April
MA : May
JN : June
JL : July
AU : August
SE : September
OC : October
NO : November
DE : December
8.2.1 Displaying the list of ingredients on the label
The Regulations prescribe how the list of ingredients must be displayed on the label [130(3)(a)-(d), CR].
- Wording: show the words "Ingredients" at the beginning of the list without any intervening written, printed or graphic material [132.14(1)(a)-(b), 132.17(1)(a)-(b), 132.21(1)(a)-(b), CR]
- Background: white
- Type colour: black
- Type style: regular weight and width standard sans serif without italics
- Type size: at least 6 points and must be smaller than the type size used for the health warning message that appears on the label
- Outset: at least 6 points on all sides
8.2.2 Naming ingredients
Cannabis extracts: Ingredients must be listed by their common or chemical name, except for vitamins which must be referred to by their chemical name [132.14(1)(c)(ii)-(iii), CR].
Cannabis topicals: Ingredients must be listed by their INCI names, or if none exists, by their chemical names, or by their EU trivial name set out in the schedule of the Cosmetic Regulations. In the case of a botanical ingredient, it must be specified at least by the genus and species portions of its INCI name, or if none exists, by its chemical name [132.17 (1)(c), CR]. For more information on the INCI system, refer to Health Canada's Guide to Cosmetic Ingredient Labelling.
Edible cannabis: Ingredients and their constituents must be listed by their common names, which means the applicable name listed in column II of Table 1 of the Common Names for Ingredients and Components Document. If not listed, for standardized foods, the name printed in boldface type in the Common Names for Ingredients and Components Document; the name prescribed by any other Regulations (e.g., SFCR); or in all other cases, the name by which the food is generally known [132.21(1)(c)(iii), CR].
Common names, chemical names of ingredients and their constituents should not be abbreviated.
8.2.3 Order of ingredients
Ingredients must be separated by a comma and shown in descending order of their proportion by weight before the ingredients are combined to form the final cannabis [132.14(1)(c)(i), 132.17(1)(c), 132.21(1)(c)(i), CR]. For cannabis extracts and cannabis topicals, any ingredient in proportion by weight less of 1% or less may be listed in any order after the ingredients that are present in a proportion of more than 1% [132.14(2), 132.17(2), CR]. Additionally, the following ingredients may be displayed at the end of the ingredients list in any order:
Cannabis extracts: Flavouring agent(s) [132.14 (3), CR]
Cannabis topicals: Fragrance or flavours listed by the term parfum or aroma [132.17(3), CR]
Edible cannabis: Ingredients referred to in B.01.008.2(4) of the FDR such as spices, seasonings, herbs (except salt), sulphites and all of their applicable common names [132.21(3), CR]
There are some ingredients that may be shown collectively in the list such as those set out in column I of Table 2 of the Common Names for Ingredients and Components Document, unless one of the ingredients referred to the table is shown separately in the list of ingredients by its common name [132.21, CR].
Important: Depending on the class of cannabis, there are a number of restricted ingredients for edible cannabis (e.g., meat products, poultry products and fish ingredients), and prohibited ingredients for cannabis extracts (e.g., added caffeine, probiotics, sugars, sweeteners) [101.3(2), CR].
8.2.4 Order and grouping of constituents
Constituents in edible cannabis must be shown in descending order of their proportion of the ingredient by weight before the constituents are combined to form the edible cannabis. Constituents must be shown together after the ingredient's common name in parentheses and be separated by a comma unless the constituents are listed as an ingredient [132.21(1)(c)(i)-(ii), 132.21(1)(d), CR].
Constituents that are required to be shown on the label include:
- Those listed in subsection B.01.009(4) of the FDR (e.g., peanut oil)
- Certain food preparation and mixtures added to a food that has one or more specific constituents listed in subsection B.01.009(3) of the FDR (e.g., salt, aspartame) [132.26(3), CR]
Constituents that are not required to be shown on the label include:
- Those listed in table B.01.009(1) of the FDR (e.g., butter, rice) [132.26 (1), CR]
- Certain food preparation and mixtures added to a food listed in the table under B.01.009(2) of the FDR (e.g., food colour preparation, spice mixtures) [132.26 (2), CR] unless the ingredients or constituents are listed under B.01.009(3), than those must be shown by their common names in the list of ingredients [132.26(3), CR]
Constituents that may be shown collectively in the list of ingredients on a label include:
- Those listed in column I of Table 2 of the Common Names for Ingredients and Components Document, unless one of the constituents referred to the table is shown separately in the list of ingredients by its common name [132.21, CR]
Example 1 - Ordering and grouping of constituents
Ingredients: Tomato paste (tomatoes, salt, benzoic acid), Sugar, Modified corn starch, Lemon juice from concentrate (water, concentrated lemon juice, sugar, benzoic acid), Water, Spices, Salt, Allura red
- The constituents "tomatoes," "salt" and "benzoic acid" are shown in descending order of proportion by weight after the ingredient "Tomato paste". They are grouped together in parentheses and are separated by a comma. This ordering and grouping of constituents was also applied to the constituents of the ingredient "Lemon juice from concentrate".
Important: The Regulations reference a number of tables in the FDR with respect to edible cannabis. While the Regulations use the term "constituent", and not "component" as per the FDR, the meaning is the same. Specifically, where the Regulations refer to FDR tables, "component" in the FDR tables is to be read as "constituent" [132.21, 132.23, 132.26, CR].
8.2.5 Order and grouping of sugars-based ingredients
Sugars-based ingredients in edible cannabis must be shown in descending order of their proportion by weight. Sugars-based ingredients must be shown together after the term "Sugars" in parentheses and be separated by a comma [132.21(1)(g), CR].
Example 2 - Displaying sugars-based ingredients
Ingredients: Sugars (fancy molasses, brown sugar, sugar), Flour, Vegetable oil shortening, Liquid whole egg, Salt, Sodium bicarbonate, Spices, Allura Red
- The sugars-based ingredients "fancy molasses," "brown sugar" and "sugar" are grouped together in descending order of proportion by weight. They are shown after the term "Sugars" in parentheses and each sugars-based ingredient is separated by a comma.
Tip: Sugars-based ingredients has the same meaning as in subsection B.01.001(1) of the FDR. The requirement to group sugars-based ingredients under the FDR is intended to help consumers understand their relative proportion in the food compared to other ingredients as well as identify unfamiliar sources of sugars in their foods. For more information on sugars-based ingredients, see CFIA's website: Grouping sugars-based ingredients.
8.2.6 Declaring food allergens, gluten and added sulphites
Food allergens, gluten and added sulphites at levels of 10 parts per million (ppm) that is present in edible cannabis must be declared by their prescribed source name or common name [132.23, 132.21(1)(f), CR]. They must be shown in one of two ways:
- In the list of ingredients (see example 3 and 4 below)
- In a food allergen source, gluten source and added sulphites statement [132.23(1) and (2), CR] (see example 5 below)
A. List of ingredients
The source of food allergens and gluten must be shown after the ingredient in parentheses, if the source:
- Is the ingredient; or
- Is present in the ingredient, but is not a constituent of or present in a constituent of that ingredient; or
- Is, or is present in, a constituent of the ingredient and the constituent is not shown in the list of ingredients [132.21(1)(e)(ii)(A)-(C), CR]
However, if the source of allergen or gluten is that constituent, or is present in that constituent, then the source must be shown after the constituent, in parentheses and be separated by a comma [132.21(1)(e)(iii) CR].
Example 3 - Displaying food allergens and gluten in the list of ingredients
Ingredients: Flour (wheat), Liquid albumin (egg), Vegetable oil, Sugar, Chocolate chips (milk) (sugar, chocolate liquor, cocoa butter, milk ingredients, soy lecithin, salt, natural flavour).
- Wheat protein is an inherent part of flour but is not a constituent. Since wheat is both a food allergen and gluten source, it must be declared. Liquid albumin is an egg protein, and, as egg is a food allergen, it must be declared. The constituents of "Chocolate chips" are declared in parentheses after the allergen source milk.
Ingredients: Pastry pieces [flour (wheat), butter (milk), liquid albumin (egg), canola oil], Sugar, Natural flavour.
- The constituents of "Pastry pieces" must be declared. Furthermore, the food allergen and gluten prescribed source names wheat, milk and egg are declared in parentheses after the constituent.
Added sulphites must be shown in parentheses after the ingredient, of which the sulphites are a constituent; or at the end of the list where they may be shown in any order with the other ingredients that are shown at the end of the list [132.21(1)(f)(iii), CR].
Example 4 - Displaying added sulphites in the list of ingredients
Ingredients: Rolled oats, Flour (wheat), Liquid whole egg, Apricot jam with pectin (sulphites), Salt, Sodium bicarbonate, Soy lecithin
- Added sulphites are shown parentheses after the ingredient "Apricot jam with pectin."
Ingredients: Rolled oats, Flour (wheat), Liquid whole egg, Apricot jam with pectin, Salt, Sodium bicarbonate, Soy lecithin, Sulphites
- Sulphites are shown at the end of the list of ingredients in any order.
B. A food allergen source, gluten source and added sulphites statement
The source of food allergens or gluten must be in a food allergen source, gluten source and added sulphites statement if the source:
- Is, or is present in, an ingredient that is not shown in the list of ingredients, but is not a constituent of that ingredient or present in a constituent of that ingredient; or
- Is, or is present in, a constituent and neither the constituent nor the ingredient in which it is present is shown in the list of ingredients [132.18(1)(l)(A), 132.19(1)(i)(A), 132.18(1)(l)(B), 132.19(1)(i)(B), 132.25(1)(d)(i)-(ii) 132.25(2), CR].
All food allergens, gluten and added sulphites information must appear in the statement at least once, even if that information is already shown in the list of ingredients [132.25(1)(d), CR].
The statement must show the words "Contains" at the beginning of the list without any intervening written, printed or graphic material and appear on the same continuous surface as the list of ingredients [132.25(1)(a)-(c), CR].
Example 5 - A food allergen source, gluten source and added sulphites statement
Ingredients: Wheat flour, Water, Vegetable oil margarine, Sugar, Yeast, Canola oil shortening, Potato starch, Garlic, Salt, Parsley, Seasoning, Diacetyl acid esters of mono & diglycerides, Whey powder, Calcium propionate, Potassium bisulphite
Contains: Wheat, Milk, Sesame, Sulphites
- The food allergen source, gluten source and added sulphites statement ("Contains") is shown after the list of ingredients. All food allergens, gluten and added sulphites are declared at least once in the statement, even though wheat and potassium bisulphite (sulphites) already appear in the ingredient list.
8.2.7 Declaring a risk of cross-contamination
Cross-contamination statements may be shown when, due to a risk of cross-contamination, the edible cannabis may contain the source of a food allergen or gluten in edible cannabis product.
These statements must not be used when an allergen or allergen-containing ingredient or constituent is deliberately added to edible cannabis. In these cases, the mandatory food allergen and gluten statement, as outlined in section 8.2.6 of this guide, is required.
The cross-contamination statement must be displayed after the food allergen source, gluten source and added sulphites statement, or, if there is none, after the list of ingredients. It must appear on the same continuous surface as the list of ingredients and the gluten source and added sulphites statement if there is one. It must not contain any intervening printed, written or graphic material between it and the list of ingredients or statement that immediately precedes it [132.24, CR].
Example 6 - A cross-contamination statement
Ingredients: White beans, Water, Sugars, Pork, Salt, Modified cornstarch, Onion powder, Mustard, Spices.
Contains: Mustard
May contain: Sesame, Soybeans
- The cross-contamination statement "May contain" is shown after the list of ingredients and after the food allergen source, gluten source and added sulphite statement.
Tip: Cannabis products containing cannabis extracts must include on the label any food allergens [132.11(i), 132.12(1)(h), CR]. While there are no specific display formats to declare these allergens, licence holders may consider following a similar format to the one listed here to declare food allergens for edible cannabis.
Important: A gluten-free claim or a claim pertaining to the absence of a specific food allergen source is permitted on the label provided that the claim is factual and not misleading. General claims stating only "allergen-free" or "no allergens" are considered to be too broad in nature and are therefore not acceptable. Refer to CFIA's guidance on gluten-free and allergen-free claims for more information.
Health Canada has no specific thresholds for gluten in cannabis products but considers levels of gluten protein below 20 ppm of not posing any health risks to consumers with celiac disease. Refer to Health Canada's Position on Gluten-Free Claims for more information.
8.2.8 Nutrition facts table for edible cannabis
The nutrition facts table must be shown for all edible cannabis products [132.18(1)(n), 132.19(1)(i), CR]. The specific information and display format are shown in Figure 4 [132.22, CR]. The presentation of the table is incorporated by reference as part of the Regulations and can be found on Health Canada's website: Directory of Nutrition Facts Table Formats for Edible Cannabis
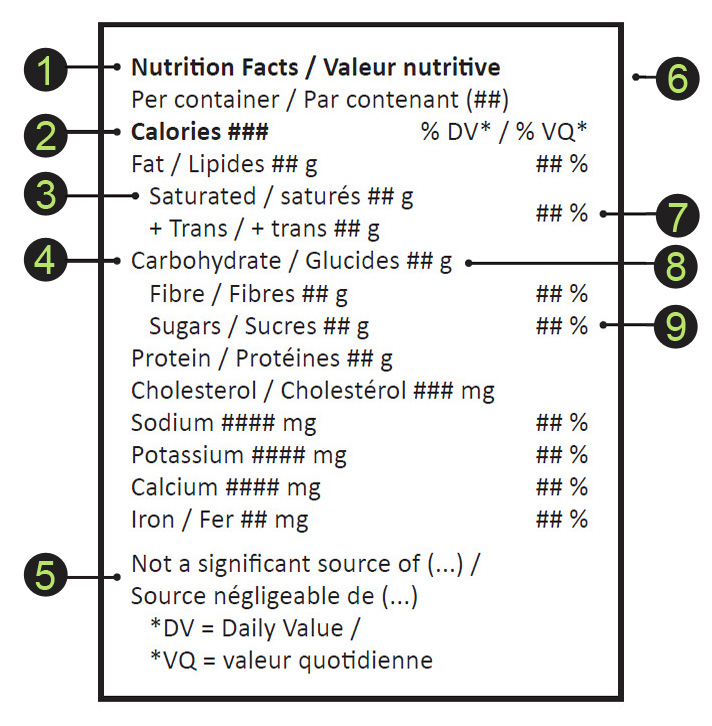
Figure 4 - Text Equivalent
8.3 Required information on small immediate containers
All immediate containers holding a cannabis product, regardless of the container's size, must display the required information (e.g., standardized cannabis symbol, health warning message, THC and CBD content, and brand name) on the PDP of the label [130(1), CR].
However, if the immediate container is too small to fit the other required product information (e.g., class of cannabis, packaging date, list of ingredients) on the PDP or the area of the label that is visible under customary conditions of purchase and use (i.e., the exterior display surface), an accordion or peel-back panel, or a label that extends beyond the display surface may be used to display this required information [132.27, CR].
In this case, the panel must meet the requirements outlined in section 132.27 of the Regulations including:
- Not be easily removed under normal use [132.27 (3)(b), CR]
- Not display a brand element [132.27(5), CR]
- Be re-sealable [132.27 (3)(a), CR]
Additionally, the label must include a statement regarding the location of the information that is not included on the label (e.g., the words lift panel). It may also include a black and white image to provide instructions on how to open the panel [132.27(6) and (7), CR].
Table 6 summarizes what and where the required information must be displayed for small immediate containers if using a peel-back panel. Refer to Table 5 in section 8.2 of this guide for details on each requirement.
| Cannabis Regulations Reference | May be displayed on the peel-back or accordion panel, or the exterior display surface | Must be displayed on the exterior display surface |
|---|---|---|
|
123(1)(a),(c)(ii),(d) |
|
|
8.4 Other information about the cannabis product
Licence holders should provide additional product information regarding the proper use of their cannabis products. This information could include:
- Directions for use of the product
- Information about the product form or formulation
- Other warnings and precautions
Any additional information (e.g., composition information such as cannabis terpenes or other cannabinoids; strain name, percentage of THC and CBD content for dried cannabis) that is included on the label must comply with the Cannabis Act and its regulations.
Other warnings and precautions outside the Regulations that are specific to the product should also be included on the label.
For example, the Cosmetic Ingredient Hotlist is an administrative tool that Health Canada uses to communicate to manufacturers and others that certain substances may be prohibited or restricted for use in cosmetics. While prohibited ingredients should not be used, restricted ingredients are permitted only if the conditions of use and/or cautionary statement(s) are met. Cannabis products containing these ingredients may be considered injurious to the health of the user of a cannabis product under the Regulations depending on the nature and intended use of the particular product. Health Canada strongly encourages licence holders to make use of the Hotlist when looking to determine whether cautionary statements are appropriate for a particular ingredient when used in cannabis topicals.
If other product information is displayed, it must be in the following format [130(8), CR]:
- Type style: regular weight and width standard sans serif without italics
- Type colour: black or white
- Type size: smaller than or equal to the type size of the information that is required to be shown on the label referred to in subsection 130(3) of the Regulations (refer to section 8.2 of this guide)
Important: Licence holders are responsible for providing appropriate directions for use and cautions about their cannabis products. They are also responsible for complying with the Act and Regulations, and other federal and provincial legislation that may apply to them or their activities.
Health Canada has a number of tools at its disposal, including ministerial orders for provision of information, measures, tests and studies, and recalls that could be used if considered necessary to address an issue of public health or public safety and/or to verify compliance or prevent non-compliance, as the case may be.
8.5 Labelling cannabis other than a cannabis product
Cannabis that does not meet the definition of a cannabis product, such as cannabis that is sold intra-industry or bulk cannabis for export that will be packaged and labelled at the destination jurisdiction, must be labelled with the following information:
- Contact information of the licence holder that sells, distributes or exports the cannabis;
- Lot number; and
- Packaging date [138, CR].
9.0 Transition period for packaging and labelling
The Cannabis Regulations as amended by the Regulations Amending the Cannabis Regulations (New Classes of Cannabis) include transitional provisions related to packaging and labelling.
Below is a summary of key milestones and dates:
On October 17, 2019 (Addition of cannabis extracts, cannabis topicals, and edible cannabis as classes of cannabis)
Cannabis extracts, cannabis topicals and edible cannabis will be added as classes of cannabis to Schedule 4 to the Act. The amended Regulations come into force.
From October 17, 2019, to 2020 (12 month transition period)
Licence holders who were authorized to conduct certain activities under the Regulations prior to October 17, 2019, will have until October 17, 2020, to adjust and align with the new packaging and labelling requirements.
Certain cannabis products (i.e., cannabis plant seeds, cannabis plants, dried cannabis, and fresh cannabis) may continue to be sold and distributed if they are packaged and labelled under certain conditions in accordance with the Regulations as they read immediately before the day on which the amendments come into force:
- With one of the health warning messages as it is in the incorporated by reference document before October 17, 2019, and they are displayed in rotation on each type of container of each brand name of the cannabis product that is packaged in a year, so that each message is displayed, to the extent possible, on equal numbers of containers of that product [77(1), CR]
- With the THC and CBD content in % values for dried and fresh cannabis [79(1), CR]
- Without the size restriction on the brand element for dried and fresh cannabis, cannabis plants and cannabis plant seeds that does not have the standardized cannabis symbol (i.e., products with a THC in a concentration of 10 μg/g or less) [80(1), CR]
- Without the immediate container limitation per outermost container for dried and fresh cannabis, cannabis plants and cannabis plant seeds [76(1), CR]
- Without the cannabis possession statement for fresh cannabis and cannabis plant seeds [78(1), CR]
For cannabis oils, licence holders, provincial/territorial retailers and other authorized persons are exempted from the application of the Regulations if they were previously authorized and the activities are conducted in accordance with the Regulations as they read immediately before October 17, 2019.
On October 17, 2020 (Removal of cannabis oil as a class of cannabis)
Cannabis oil will no longer exist as a class of cannabis from Schedule 4 to the Act. While cannabis oil will cease to exist as a standalone class of cannabis on this date, cannabis oil products will continue to be permitted for sale within the new classes of cannabis (i.e., cannabis extracts, cannabis topicals, and edible cannabis).
October 17, 2020, and beyond
Only persons other than the licence holder are authorized to continue depleting their inventory under certain conditions.
10.0 Contact us
If you have general questions about the Cannabis Act and its regulations, you can reach Health Canada by email cannabis@canada.ca or phone at 1-866-337-7705.
11.0 Feedback-Help us improve
Health Canada is committed to providing all stakeholders with timely, accurate and reliable information. This includes providing information needed to comply with the Cannabis Act and its regulations. We would appreciate receiving your feedback on whether this guide was useful, and we welcome your suggestions for improvement. Email your feedback to us at cannabis@canada.ca and indicate in the subject line Feedback on the Packaging and labelling guide for cannabis products.
Appendix A: A cannabis product that meets the packaging and labelling requirements
An example of edible cannabis in discrete units
- The standardized cannabis symbol
- The brand name of the cannabis product
- THC and CBD content
- Health warning message
- Other brand element
- Other required information about the cannabis product
- Non-required information about the cannabis product
- Nutrition facts table
- List of ingredients
- Bar code


Figure 5 - Text Equivalent
Report a problem or mistake on this page
- Date modified:
